Background: Autologous stem cell transplantation (ASCT) is an effective treatment for patients with AL amyloidosis. In recent years, due to the advent of daratumumab-based regimens that achieve fast, deep, and long-lasting hematological responses, the role of ASCT and its optimal timing have been put in discussion. Access to ASCT in Latin America is difficult and specialized transplant centers are scarce, as is access to daratumumab-based chemotherapy regimens.
Objectives: To describe the characteristics and evolution of patients with AL amyloidosis who received an autologous stem cell transplantation (ASCT) estimating organic response, overall and progression-free survival. To compare the evolution between patients initially eligible for transplantation and those who were not.
Methods: We designed a retrospective cohort study of adult patients with AL Amyloidosis who received an ASCT between 2012 and 2022. Those who did not complete one year of follow-up were excluded. We obtained data from patients enrolled in the Institutional Amyloidosis Registry. Continuous variables were compared with the Student or Mann-Whitney test according to distribution, and categorical variables were compared with chi2 or Fisher. Overall and progression-free survival was estimated using Kaplan Meier.
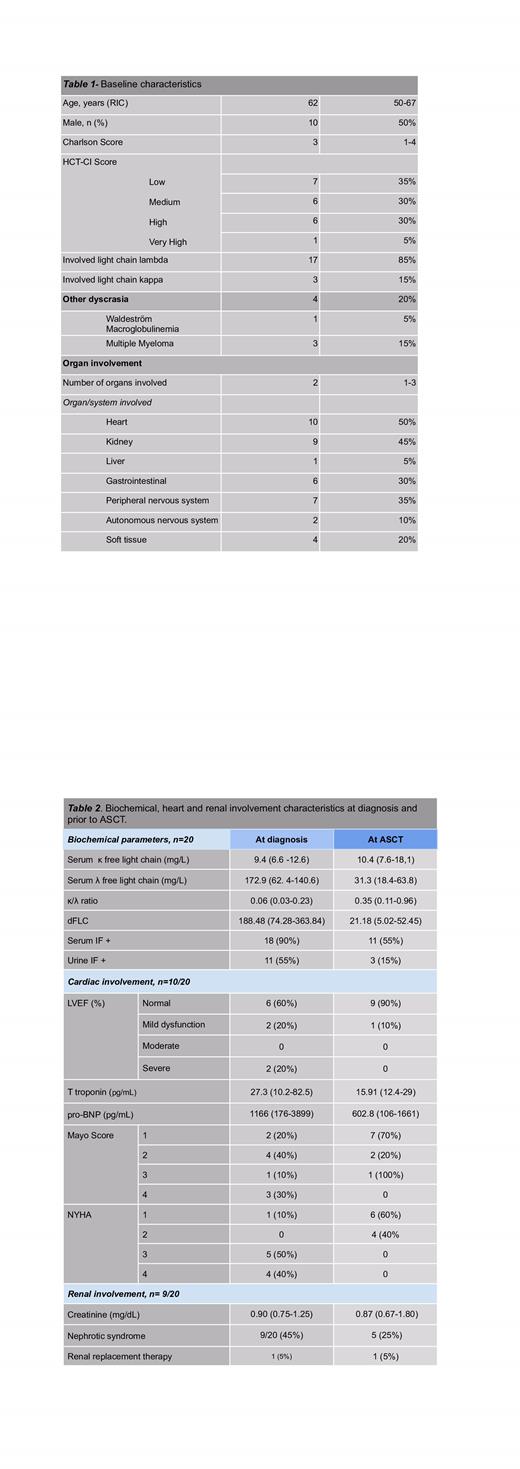
Results: Of 129 patients with AL, 32 received ASCT (24.5%). Twenty were eligible for analysis.The baseline characteristics are shown in table 1.
The median time to diagnosis was 14 months (IQR 7-27). At the time of diagnosis, 9 patients (45%) were not eligible for transplantation; in all cases, ineligibility was due to cardiac involvement, with 4 receiving heart transplantation prior to ASCT. The longest time between heart transplantation and ASCT was 348 days. More information on renal and cardiac involvement at diagnosis and at ASCT is shown on table 2.
Regarding treatment, 18 patients underwent a single line of chemotherapy with a median of 4 cycles prior to ASCT. All patients received regimens with bortezomib and only 3 added daratumumab. One patient underwent ASCT without hematological response, 7 with a partial response (PR), 5 with very good partial response (VGPR) and 7 with a complete response (CR). The time to best hematological response was not statistically different between patients who did and did not receive daratumumab, probably due to the low number of patients in the daratumumab group.
The median age at transplant was 63.5 years old (IQR 50.5-68). Fourteen patients underwent conditioning with full dose melphalan and 6 with reduced dose. There was no statistically significant difference between these groups in terms of relapse and survival. The median number of days of hospitalization for the transplant was 20 (IQR 18-24). Only two patients required intensive care. The median number of transfusions per patient was 5 (IQR 3-9). There were no deaths during hospitalization or in the first 100 days of followup.
The median time to engraftment was 12.5 days (IQR 11-14); in patients with cd34 collects > 6.5x10(6), median time to engraftment was 12.2 days (IQR 10.6-13.8), and in patients with cd34< 6.5x10(6), 15.4 days (IQR 9.9-20.9). This difference, as well as the difference in transfusions among the two groups, was not statistically significant.
After ASCT, 15 patients obtained CR and 5 VGPR; 8 patients relapsed. The median time to relapse was 6.10 years (IQR 2.28-6.70).
Of 9 patients with renal involvement, 7 presented renal response (2 pre and 5 post ASCT). Of the remaining, 1 received a post ASCT kidney transplant. Median time to renal response from start of treatment was 740 days (IQ 400-1736). Amongst the 10 patients with cardiac involvement, 4 underwent pre-ASCT heart transplantation. Of the remaining, 5 (83%) documented cardiac responses with a median of 538 days (IQR 487-611), in 2 cases prior to ASCT. There were no differences in morbidity and mortality between heart transplant recipients and the ones who did not receive a heart transplantation.
Only 3 patients died, 2 from causes related to cardiac involvement and one from unrelated causes.
Conclusions: ASCT is a safe treatment when performed in an experienced center. It ensures deep responses and prolongs progression-free survival. Patient selection is key to achieve these outcomes. Ineligibility at diagnosis is not related to worse post ASCT morbidity and mortality outcomes. Heart transplantation prior to ASCT is a safe strategy to favor eligibility.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal